 Nitinol is a unique metallic alloy that is ideally suited to use in medical devices. It has excellent biocompatibility and most of all it has the ability to undergo significant recoverable deformations. This allows devices made with Nitinol to be reduced to catheter dimensions and expanded at the implant site.
Nitinol is a unique metallic alloy that is ideally suited to use in medical devices. It has excellent biocompatibility and most of all it has the ability to undergo significant recoverable deformations. This allows devices made with Nitinol to be reduced to catheter dimensions and expanded at the implant site.
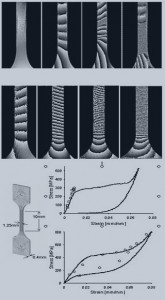
Much work has been done to characterize Nitinol from a materials science perspective. It is a multi-phase material with a parent and a transformed phase. In its most common application, the parent phase is transformed under the application of stress to yield stress-induced martensite. This results in a stress-strain curve with a plateau that is primarily a function of the austenite transformation temperature of the material. Complications arise because the material has multiple phases, the loading curve involves hysteresis, the material response is history dependent and the material is particularly sensitive to processing conditions.
Finite Element Analyses of Nitinol medical implants require the analyst to choose a material model and calibrate that material model. This poses a challenge as a comprehensive description of Nitinol’s unique response remains incomplete. The choices range from complex micromechanical models that involve keeping up with 18 or more crystallographic representations to simplified phenomenological models that capture the austenite loading slope, plateau and martensite loading slope. Some models capture the difference between tension and compression behavior, some can accomodate shake-down effects and others cover both superelastic and shape memory characteristics.
There is no clear answer as to which material model is most appropriate for a given application. It all depends on the purpose of the analysis. For many applications involving predicting the safe fatigue life of a component, generally, a simple tri-linear phenomenological model that is calibrated appropriately is sufficient and appropriately conservative. In cases where shape memory recovery is involved, a more sophisticated material model would be required.
 The more important questions to ask and answer depend on the calibration of the material model and the application to the in-use conditions. This includes both the stress-strain of force-deflection behavior as well as the material limit criteria. Because Nitinol is so sensitive to processing history it is generally far more effective to establish a self-consistent quality program based on readily measurable parameters than to involve complex representations of constitutive behavior with inadequate calibration. In this way, statistics and experience can help strengthen the confidence in engineering judgments made in the course of developing implantable medical devices.
The more important questions to ask and answer depend on the calibration of the material model and the application to the in-use conditions. This includes both the stress-strain of force-deflection behavior as well as the material limit criteria. Because Nitinol is so sensitive to processing history it is generally far more effective to establish a self-consistent quality program based on readily measurable parameters than to involve complex representations of constitutive behavior with inadequate calibration. In this way, statistics and experience can help strengthen the confidence in engineering judgments made in the course of developing implantable medical devices.
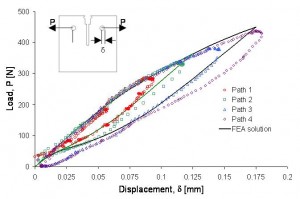
Complex loading conditions involving superimposed torsion, bending, axial extension and compression challenge ALL material fatigue theories. Medical devices are unique in that they are architectural structures where designers and engineers need to assure safe lifetimes with an incredibly low tolerable failure rate. A common approach that has gained much acceptance is to utilize a calibrated material model for Nitinol and a Finite Element Analysis of a fatigue coupon sample to generate a fatigue limit in terms of local strain versus number of cycles to failure. While this is only a one-dimensional approach, it has proven quite useful and can be implemented with readily available test equipment.
While much work remains to be done to develop, validate and generalize predictive methods for evaluating the safe lifetime for implantable devices, there are many successful examples of self-consistent approaches for specific device families.
Some general guidelines to follow are:
1) Start simple and add complexity to your model step by step.
2) Validation is a process: go back and forth between computational and experimental result.
3) Bound all unkown parameters and do systematic studies to identify sensitive factors.
4) Remember: “The purpose of computing is insight, not numbers”. Learn as you go and keep the focus on improving the design and manufacturing of the product.
