 For medical devices, rarely does “one size fit all”. The human body is extremely variable and patient populations, especially those with disease present a wide range of differences that the medical device design engineer must consider.
For medical devices, rarely does “one size fit all”. The human body is extremely variable and patient populations, especially those with disease present a wide range of differences that the medical device design engineer must consider.
Identifying the worst case size for a product family is an important part of the validation process for implantable medical devices. Understanding your product and especially the in vivo loading conditions are essential for engineering the structure and material specifications for each size over the intended product range.
Typically, there are numerous sources of nonlinearity associated with implantable medical device design. The materials we use, the geometries and especially the physiology we treat all respond in ways that are difficult to describe in simple terms. It is tempting to design a product by considering an idealized patient population and then simply “scale” that design to smaller and larger sizes. But this approach can can result in a poorly optimized product family. Furthermore, when one considers device/lumen interaction and the resulting compliance under physiological loads, identifying the worst case loading condition is not a straightforward activity.
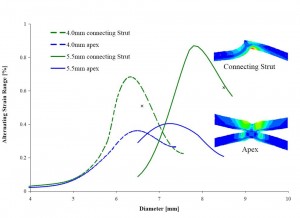
The figure above illustrates how the alternating fatigue strain for a stent-like product can vary for deployment to different diameters. It is based on an analytic model of lumen compliance and finite element analysis models of the two device sizes. Clearly, the results indicate a highly nonlinear system that precludes the selection of a single worst case device size and implant condition based on the “four corners” approach. Assuming that the largest device put into the smallest lumen will result in the most challenged loading condition is naive.
When it is possible, it is preferred to model ALL device sizes to determine the worst case size. It is also advantageous to develop and validate a simulated model of the intended physiology for the implant and use this model to verify the performance of each size in a design family. Parameters such as radial force, anchoring, dynamic compliance, vessel tortuosity and fatigue loading conditions can then all be evaluated for each device size and safety established for the complete instructions for use (IFU) for the product.
